
钱旭红,男,博士、院士、国家自然科学基金咨询委员(化学部)、英国皇家化学会会士、德国洪堡基金会大使科学家。 1962年生于江苏宝应。1978.9 进入华东化工学院(现华东理工大学)石油化工系,1982.7, 获基本有机化工学士学位,1982.9 华东化工学院(现华东理工大学)精细化工系研究生,1985.4获硕士学位, 1988.7华东理工大学获工学博士学位;1989.8-1990.8美国德克萨斯州拉玛大学research associate; 1990.9-1991.12德国维尔茨堡大学洪堡基金(avh)博士后。1988-1992.4华东理工大学讲师;1992.8-1994.7副教授;1994.8-至今,教授;1995.12博士生导师; 1986-1987;华东理工大学精细化工系党总支副书记;1992.8-1995.4先后任华东理工大学精细化工学科主任、药物化工研究所所长;1995.5-7,国家高级教育行政学院学员;1995.9-1996.2任华东理工大学校长助理、1996.3-2000.10任副校长;2000.9-2004.6,大连理工大学教育部*奖励计划特聘教授(2000年获国家杰出青年科学基金)。2002.1-2010.1 上海市化学生物学重点实验室主任(兼);2003.3-2006 国家南方农药创制中心(上海) 主任(兼)。2004.7- , 华东理工大学校长。2007. 4-, 中国化工学会副理事长;2007-2009,亚洲及太平洋化工联盟主席。
获1992霍英东基金会高校优秀青年教师奖; 1995全国优秀教师;1996国务院政府特殊津贴;1997入选国家百千万人才工程;1998 中国青年科技奖;1999第六届上海十大科技精英. 作为第一完成人,获得1998、2002、2003教育部科技进步一等奖、2008上海市自然科学一等奖、2008国家科技进步二等奖、2010上海市自然科学二等奖。2011年当选中国工程院院士。
钱旭红教授的研究方向是生物有机化学与工程。其研究工作主要为由杂环芳烃和有机氟衍生的绿色化学农药、生物功能染料的化学生物学及工程。涉及农药先导、荧光传感器、抗癌先导、人工核酸酶、细胞激活剂的芳香杂环分子设计、合成、构效关系及生物应用。
研究方向
1.染料的化学生物学
其研究主要集中在荧光传感器与新机制抗癌先导,包括以下内容:
荧光传感器:由萘、萘酰亚胺、香豆素、氟硼吡咯、聚合物衍生的高选择性过渡及重金属离子(汞、铜、锌、银、钯等)荧光传感器;某些阴离子、酶的荧光传感器;以及其它的荧光探针、荧光标记。
抗癌先导:由硫化合物、噻类芳香杂环、羰酰亚胺、萘酰亚胺、萘内酰胺、苯并吲哚酚嗪、吖啶、苊萘醌、苊萘吡咯羰腈、酞菁等衍生的抗癌试剂,bcl蛋白与细胞调亡剂,拓扑异构酶抑制剂,光动力治疗剂,色素的药物化学(抗肿瘤先导、dna嵌入剂、细胞调亡剂)。
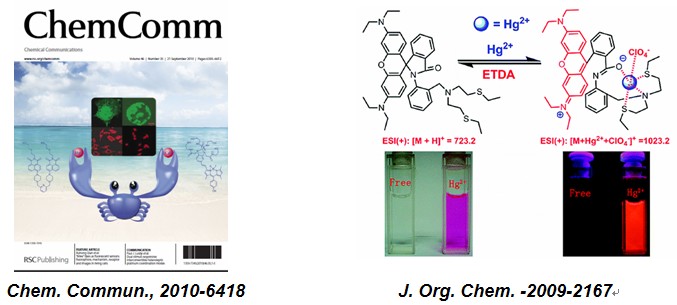
chem. commun.-2010-6418:
chem. commun.-2010-7121:
chem. commun.-2010-2772:
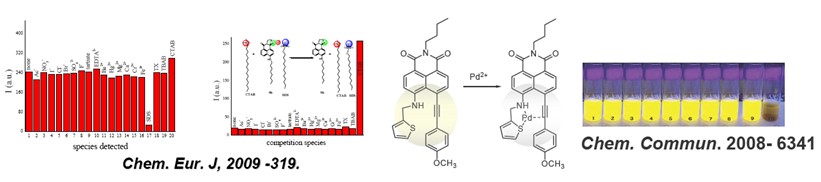
chem. commun. -2008-6339: highly sensitive and selective pd2 sensor of naphthalimide derivative based on complexation with alkynes and thio-heterocycle
chem. commun. -2008-4141: multiple molecular logic functions and molecular calculation facilitated by surfactant with its versatility
chem. commun. -2008-777: bulky 4-tritylphenylethynyl substituted boradiazaindacene: pure red emission, relatively large stokes shift and inhibition of self-quenching
chem. commun. -2008-1780: a design concept of long wavelength fluorescent analogs of rhodamine dyes: replacement of oxygen with silicon atom
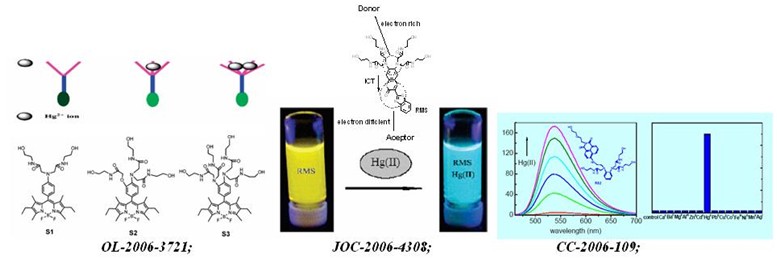
chem. commun. -2006-109: two regioisomeric and exclusively selective hg(ii) sensor molecules composed of a naphthalimide fluorophore and an o-phenylenediamine derived triamide receptor
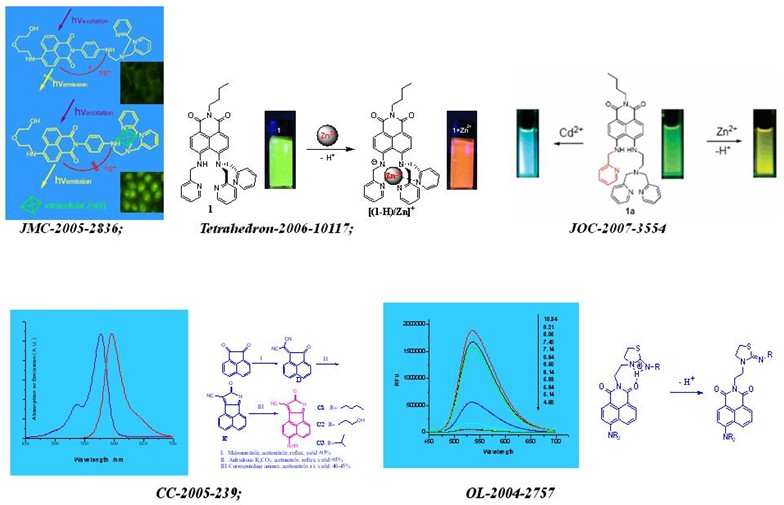
chem. commun. -2005-239: a new class of long-wavelength fluorophores: strong red fluorescence, convenient synthesis and easy derivation
chem. commun. -2001-2656: intramolecular aromatic 1,5-hydrogen transfer in preparation of oxacyclic naphthalic anhydride via unusual pschorr cyclisation
j. am. chem. soc.-2010-601:
j. am. chem. soc. -2008-16160: a highly sensitive and selective off-on fluorescent sensor for cadmium in aqueous solution and living cell
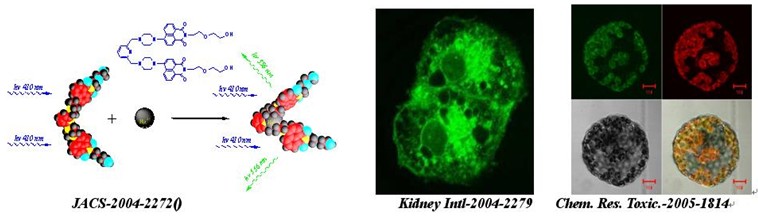
j. am. chem. soc. -2004-2272: a highly selective and sensitive fluorescent chemosensor for hg2 in neutral buffer aqueous solution
angew. chem. intl. ed. -2008-8025: a ratiometric fluorescent probe based on fret for imaging hg2 in living cells
chem. eur. j.-2010-5013:
chem. eur. j.-2010-104:
chem. eur. j. -2009-319:
chem. eur. j. -2007-7543: micelle-induced versatile performance of amphiphilic intramolecular charge-transfer fluorescent molecular sensors
org. lett. -2011-264:
org. lett.-2009-2808:
org. lett. -2008-473: ratiometric and water-soluble fluorescent zinc sensor of carboxamidoquinoline with an alkoxyethylamino chain as receptor
org. lett. -2008-29: highly efficient energy transfer in the light harvesting system composed of three kinds of boron-dipyrromethene derivatives
org. lett. -2008-633: isomeric boron-fluorine complexes with donor-acceptor architecture: strong solid/liquid fluorescence and large stokes shift
org. lett. -2008-633: 2,3,6,7-tetraamino-9,9-bis(2-ethyl- hexyl)fluorene: new multifunctional monomer for soluble ladder-type conjugated molecules and polymers
org. lett.-2006-3721: a series of polyamide receptor based pet fluorescent sensor molecules: positively cooperative hg2 ion binding with high sensitivity
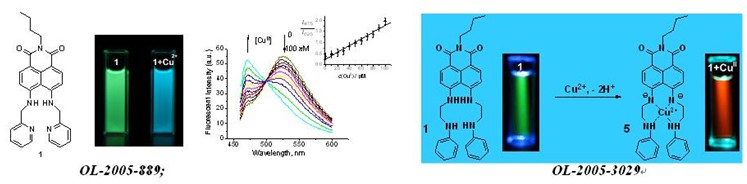
org. lett. -2005-3029: colorimetric and ratiometric fluorescent chemosensor with a large red-shift in emission: cu(ii)-only sensing by deprotonation of secondary amines as receptor conjugated to naphthalimide fluorophore
org. lett. -2005-889: ratiometric and selective fluorescent sensor for cu-ii based on internal charge transfer (ict)
org. lett. -2004-2757: novel fluorescent ph sensors based on intramolecular hydrogen bonding ability of naphthalimide
j. org. chem.-2010-3007:
j. org. chem. -2009-2167:
j. org. chem. -2008-1571: novel fluorescent fluorine-boron complexes: synthesis, crystal structure, photoluminescence, and electrochemistry properties
j. org. chem. -2007-3554: ratiometric and highly selective fluorescent sensor for cadmium under physiological ph range: a new strategy to discriminate cadmium from zinc
j. org. chem. -2006-4308: detecting hg2 ions with an ict fluorescent sensor molecule: remarkable emission spectra shift and unique selectivity
mol. cancer res.-2010-1619:
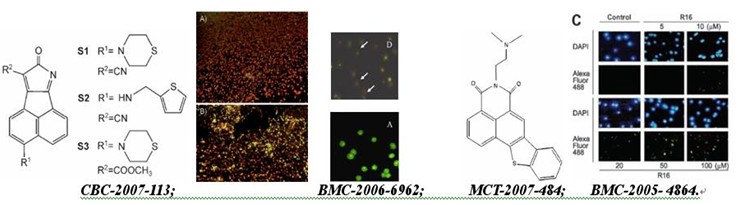
mol. cancer ther. -2007-484: r16, a novel amonafide analogue, induces apoptosis and g2-m arrest via poisoning topoisomerase ii
j. med. chem.-2010-2589:
j. med. chem. -2009-763:
kidney international -2004-2279: fluorescent imaging of acute mercuric chloride exposure on cultured human kidney tubular epithelial cells
chem. res. tox.-2009-483:
chem. res. toxic. -2005-1814: visible study of mercuric ion and its conjugate in living cells of mammals and plants
chembiochem -2007-113: novel bcl-2 inhibitors: discovery and mechanism study of small organic apoptosis-inducing agents
cancer letters-2009-193:
neoplasia-2009-1226:
eur. j. med. chem.-2009-4674:
bioorg. med. chem. lett. -2006-1562: synthesis and evaluation of novel 8-oxo-8h-cyclopenta[a]acenaphthylene-7-carbonitriles as long-wavelength fluorescent markers for hypoxic cells in solid tumor
bioorg. med. chem. lett. -2005-5909: novel synthetic isoquinolino[5,4-ab]phenazines: inhibition toward topoisomerase i, antitumor and dna photo-cleaving activities
bioorg. med. chem. lett. -2005-4864: novel thiazonaphthalimides as efficient antitumor and dna photocleaving agents: effects of intercalation, side chains, and substituent groups
bioorg. med. chem. lett. -2005-4864: synthesis, antitumor evaluation and dna photocleaving activity of novel methylthiazonaphthalimides with aminoalkyl side chains
bioorg. med. chem. lett. -2005-1139: five-member thio-heterocyclic fused naphthalimides with aminoalkyl side chains: intercalation and photocleavage to dna (ss)
bioorg. med. chem. lett. -2003-5427: novel naphthalimide hydroperoxide photonucleases: the role of thiocyclic-fused area and the difference in spectra, photochemistry and photobiological activity
bioorg. med. chem.-2010-3279:
bioorg. med. chem.-2009-7615 :
bioorg. med. chem.-2009-592:
bioorg. med. chem. -2007-1356: novel antitumor agent family of 1h-benzo[c,d]indol-2-one with flexible basic side chains: synthesis and biological evaluation
bioorg. med. chem. -2006-6962:acenaphtho[1,2-b]pyrrole derivatives as new family of intercalators: various dna binding geometry and interesting antitumor capacity
bioorg. med. chem. -2006-4639: design, synthesis, and antitumor evaluation of novel acenaphtho[1,2-b]pyrrole-carboxylic acid esters with amino chain substitution
bioorg. med. chem. -2005-3149: novel heterocyclic family of phenyl naphthothiazole carboxamides derived from naphthalimides: synthesis, antitumor evaluation, and dna photocleavage
bioorg. med. chem. -2005-1615: thio-heterocylic naphthalimides with aminoalkyl side chains: novel alternative tools for photodegradation of genomic dna without impairment on bioactivities of proteins
int. j. bio. macromol. -2006-59: study on the interaction between 4-(2-diethylanuno- ethylamino)-8-oxo-8h- acenaphtho [1,2-b]pyrrole- 9-carbonitrile and dna by molecular spectra
tetrahedron lett.-2009-22:
tetrahedron lett. -2005-6289: a proton sponge-based fluorescent switch
tetrahedron lett. -2004-3969: a novel chromatism switcher with double receptors selectively for ag in neutral aqueous solution: 4,5-diaminoalkeneamino-n-alkyl-1,8-naphthalimides
tetrahedron lett. -2003-2087: novel highly efficient fluoroionophores with a peri-effect and strong electron-donating receptors: tict-promoted pet and signaling response to transition metal cations with low background emission
tetrahedron lett. -2003-795: promoting effects of the hydroxymethyl group on the fluorescent signaling recognition of anions by thioureas
tetrahedron lett. -2002-2991: 4-amino-1,8-dicyanonaphthalene derivatives as novel fluorophore and fluorescence switches: efficient synthesis and fluorescence enhancement induced by transition metal ions and protons
tetrahedron lett. -2002-2995: synthesis and properties of benzothioxanthene dicarboximide hydroperoxide: an efficient 'time-resolved' dna photocleaver with long-wavelength
tetrahedron lett. -2001-6175: n-aroyloxynaphthalimides as novel highly efficient dna photocleavers: substituent effects
tetrahedron lett. -2000-7711: novel and highly efficient dna photocleavers: hydroperoxides of heterocyclic-fused naphthalimides
tetrahedron-2009-8099:
tetrahedron -2006-10117: exploiting the deprotonation mechanism for the design of ratiometric and colorimetric zn2 fluorescent chemosensor with a large red-shift in emission
tetrahedron -2005-11895:novel dna bis-intercalators of isoquinolino[4,5-bc]acridines: design, synthesis and evaluation of cytotoxic activity
tetrahedron -2005-11264: versatile acenaphtho[1,2-b]pyrrol-carbonitriles as a new family of heterocycles: diverse snar(h)h reactions, cytotoxicity and spectral behavior
tetrahedron -2005-8717: synthesis, antitumor and dna photocleaving activities of novel naphthalene carboxamides: effects of different thio-heterocyclic rings and aminoalkyl side chains
tetrahedron -2005-6634: synthesis of thiazo- or thiadiazo- naphthalene carboxamides via mercuric intermediates and their antitumor and dna photocleavage activities
new j. chem. -2008-472: chromogenic and fluorescent chemodosimeter for fluoride ion based on
novel anion-catalyzed intramolecular hydrogen transfer
new j. chem. -2003-337: a polyamidoamine dendrimer with peripheral 1,8-naphthalimide groups capable of acting as a pet fluorescent sensor for metal cations
new j. chem. -2002-920: novel heterogeneous pet fluorescent sensors selective for transition metal ions or protons: polymers regularly labelled with naphthalimide
dyes pigments-2009-127:
dyes pigments -2004-17: synthesis and peroxidase-staining properties of novel water soluble polyhydroxylalkyl benzidine dyes
dyes pigments -2004-9: benzothioxanthene dyes as fluorescent label for dna hybridization: synthesis and application
dyes pigments -2002-247: synthesis and properties of oligonucleotides containing fluorescent ethenodeoxyadeno sine and ethenodeoxycytidine
dyes pigments -2001-51: absorption and fluorescence spectral properties of tetra (fluoroalkoxy) metallophthalocyanines
dyes pigments -2001-43: oxazolonaphthalimides and their hydroperoxides: photophysical and photobiological properties
j. fluorine chem. -2002-161: synthesis and photosensitizing properties of fluoroalkoxyl phthalocyanine metal complexes
j. fluorine chem. -2000-69: synthesis of fluorine-containing oxazolonaphthalimide hydroperoxides as dna photocleavers
j. photochem. photobio. a -2010-86:
j. photochem. photobio. a -2010-24:
j. photochem. photobio. b -2001-35:tetra-trifluoroethoxyl zinc phthalocyanine: potential photosensitizer for use in the photodynamic therapy of cancer
j. photochem. photobio. a -2009-181:
j. photochem. photobio. b -2006-221: isoquino[4,5-bc]acridines: design, synthesis and evaluation of dna binding, anti-tumor and dna photo-damaging ability
dalton transactions-2010-7114:
dalton transactions-2010-1316:
dalton transactions-2009-1761:
org. biomol. chem.-2009-129:
j. chem. soc.-perkin 2 -2000-715: interaction of naphthyl heterocycles with dna: effects of thiono and thio groups
chem. lett. -2005-696: novel naphthalimide fluorescent sensors selective for certain proteins on basis of non-covalent interactions between enzyme and inhibitor
chinese chem. lett. -2004-118: anthracylmethyl benzoazacrown ether as selective fluorescence sensors for zn2
analy. chim. acta 2009-227:
chinese j. anal. chem. -2004-837:studies on the room temperature phosphorescence of halogen naphthalic anhydrides
j. chem. tech. biotech. -2009-1051:
sci. china b-chem.-2009-771:
j. mater. chem. -2010-10755:
j. mater. chem. -2005-2836: a ph-resistant zn(ii) sensor derived from 4-aminonaphthalimide: design, synthesis and intracellular applications
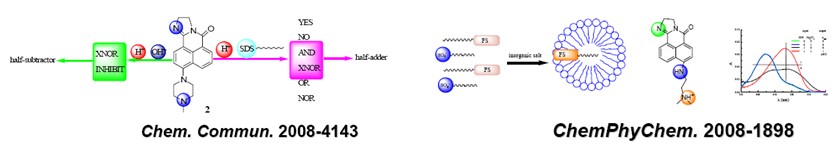
chemphyschem -2008-1891: molecular logic operations based on surfactant nano-aggregates: premicelle, micelle and its formation induced by inorganic salt
polymer -2002-5731: synthesis and photophysical properties of 1,8-naphthalimide-labelled pamam as pet sensors of protons and of transition metal ions
heterocycl. commun. -2003-229: a new class of dna intercalator and photocleaver: bis-naphthailimides with bromo and nitro substituents
monatsh. chem.-2010-89: 2.农药的化学与生物学
其研究主要集中在芳香杂环或氟化合物衍生的农药先导,包括以下内容:
昆虫调节剂与杀虫剂先导:针对几丁质合成抑制、蜕皮激动、海藻糖酶抑制、拒食、飞行调控的昆虫生长调节剂与行为调节剂,烟碱受体激动类杀虫剂。
新杂环化合物:主要有具有农药活性的氟化合物、氟烷氧基化合物、硫化合物、恶二唑、噻二唑、哒嗪酮、新烟碱、环亚胺、二芳酰基肼等。
bioorg. med. chem. lett. -2009-332:
bioorg. med. chem. lett. -2008-6513:cis-nitromethylene neonicotinoids as new nicotinic family: synthesis, structural diversity, and insecticidal evaluation of hexahydroimidazo[1,2-a]pyridine
bioorg. med. chem. -2008-7565:1,3,4-oxadiazole-3(2h)-carboxamide derivatives as potential novel class of monoamine oxidase (mao) inhibitors: synthesis, evaluation, and role of urea moiety
eur. j. med. chem.-2009-2985:
eur. j. med. chem.-2009-2113:
org. biomol. chem.-2010-2386:
tetrahedron lett.-2010-4866:
tetrahedron lett.-2009-1982:
tetrahedron-2011-285:
tetrahedron-2009-10377:
tetrahedron -2008-3877:solvent-free ugi four-component condensation: application to synthesis of philanthotoxins-12 analogues
synlett. -2008-1341: ytterbium (iii) perfluorooctanoate-catalyzed one-pot three-component synthesis of fully substituted pyrazoles under solvent-free conditions
synlett. -2008-3058: one-pot synthesis of trifluoromethyl-containing pyrazoles via sequential yb(pfo)3-catalyzed three-component reaction and ibx-mediated oxidation
j. agri. food chem.-2010- 2690:
j. agri. food chem.-2010- 2696:
j. agri. food chem.-2010- 2613:
j. agri. food chem.-2010- 2736:
j. agri. food chem. -2009-951:
j. agri. food chem. -2007-2288: synthesis, insecticidal activity, and qsar of novel nitromethylene neonicotinoids with tetrahydropyridine fixed cis configuration and exo-ring ether modification
j. agri. food chem. -2006-125: synthesis and herbicidal activity of novel 3-aminocarbonyl-2-oxazolidinethione derivatives containing a substituted pyridine ring
j. agri. food chem. -2006-8793: novel, unnatural benzo-1,2,3-thiadiazole-7-carboxylate elicitors of taxoid biosynthesis
j. agri. food chem. -2005-3120: syntheses, antifeedant activity, and qsar analysis of new oxa(thia)diazolyl 3(2h)-pyridazinones
j. agri. food chem. -2003-152: synthesis and antifeedant activity of new oxadiazolyl 3(2h)-pyridazinones
j. agri. food chem. -2001-124: synthesis and quantitative structure-activity relationships of new 2,5-disubstituted-1,3,4-oxadiazoles
j. agri. food chem. -2001-5279: synthesis and quantitative structure-activity relationships of fluorine-containing 4,4-dihydroxylmethyl-2-aryl imino- oxazo (thiazo)lidines as trehalase inhibitors
j. agri. food chem. -1999-4415: quantitative studies on structure-activity relationship of sulfonylurea and benzoylphenylurea type pesticides and their substituents' bioisosterism using synthons' activity contribution
j. agri. food chem. -1996-1538: molecular modeling study on the structure-activity relationship of substituted dibenzoyl-1-tert-butylhydrazines and their structural similarity to 20-hydroxyecdysone
pest management sci.-2010-238:
pesticide biochem. physiology -2004-42: photolarvicidal effect of thienyl 1,3,4-thia(oxa)diazoles and their potential dna photocleavage
acs symposium series -2005(892)-273: oxadiazole derivatives as novel insect-growth regulators: synthesis and structure-bioactivity relationship
j. fluorine chem. -2006-182: synthesis and herbicidal activities of fluorine-containing 3-pyridylmethyl-2- phenyliminothiazolidine derivatives
j. fluorine chem. -2005-297: synthesis and fungicidal activity of fluorine-containing phenylimino-thiazolidines derivatives
j. fluorine chem. -2005-53: synthesis and biological activities of hydroxyl-protected fluorine-containing 4,4-dihydroxylmethyl-2-aryl-iminothiazolidines
j. fluorine chem. -2004-1159: synthesis and fungicidal activity of fluorine-containing phenylimino-thiazolidines derivatives
j. fluorine chem. -2004-1609: syntheses, structures and bioactivities of fluorine-containing phenylimino-thia(oxa)zolidine derivatives as agricultural bioregulators
j. fluorine chem. -2003-163: synthesis and insecticidal activities of novel 2,5-disubstituted 1,3,4-oxadiazoles
j. fluorine chem. -2003-51: synthesis and insecticidal activity of new substituted n-aryl-n’-benzoylthiourea compounds
j. fluorine chem. -2002-63: syntheses and insecticidal activity of new 2-(5-(trifluoromethyl)pyridyloxymethyl) -1,3,4-oxadiazoles]
j. fluorine chem. -2000-111: fluorine containing heterocyclic compounds: synthesis of 6-substituted-2-substituted-aryl-1,2,4-triazolo[5,1-b] 1,3,5- thia- diazin-7-one derivatives
j. fluorine chem. -2000-173: syntheses and insecticidal activities of novel 2-fluorophenyl-5-aryl/cyclopropyl-1,3,4-oxadiazoles
j. fluorine chem. -2001-143: synthesis and biological activities of fluorine-containing n, n’-diphenylcarbamimidothioates
chem. phys. lett. -2003-489: intramolecular noncovalent force in cyclic amidines: nonbonded interaction between carbon atoms and heteroatoms
pest. manag. sci. -2003-933: the toxic and anti-feedant activity of 2h-pyridazin-3-one-substituted 1,3,4-oxadiazoles against the armyworm pseudaletia separata (walker) and other insects and mite
carbohydrate research -2001-79: syntheses and activities as trehalase inhibitors of n-arylglycosylamines derived from fluorinated anilines
can. j. chem. -2003-272: n'-tert-butyl-n'-aroyl-n-(alkoxycarbonylmethyl)-n- aroylhydrazines, a novel nonsteroidal ecdysone agonist: syntheses, insecticidal activity, conformational and crystal structure analysis
j. chem. res. -2006- 626: synthesis and bioactivities of novel neonicotinoids dioxolane compounds
j. chem. res. -2000-88: the crystal structure of 1-phenyl-3-methyl-5-[2 '-(4 ''-phenylmethoxylphenyl)ethoxyl]pyrazole, a potential insect juvenile hormone mimic
j. chem. res. -1999-66: the crystal structure of n-(5-phenyl-1,3,4-oxadiazol-2-yl)-n '-benzoyl urea, a novel insect-growth regulator
j. chem. res. -1998-478: crystal structure of 1-(3,5-dichloro-2,4-difluorophenyl)-3- (2,6-difluorobenzoyl)urea, an inhibitor of chitin synthesis
arkivoc -2003-141: synthesis and insecticidal activity of 1,2,4-triazole derivatives
chinese chem. lett. -2004-7: novel analogues of -terthienyl,thienyl 1,3,4-thia(oxa)diazoles as potential photoactivated insecticides:synthesis and bioactivity
chem. lett. -2001-54: a novel and practical amination of 4,5-dichloropyridazin-3-ones via reduction with hydrazine hydrate
org. prep. procedure intl. -2000-571: a facile synthesis of aryl isothiocyanates from arylamines
mol. diversity-2010-501:
monatsh. chem.-2010-1117:
sci. china-chem.-2010-1509:
phosphorus sulfur and silicon-2009-1825:
heteroatom chem.-2009-418:
j. comput. chem. -2010-586:
green chem.-2009-1414:
3.化学生物技术与工程
其研究主要集中在人工核酸酶、细胞激活剂和生物转化,包括以下方面:
人工核酸酶:由萘酰亚胺、硫杂环衍生的人工光核酸酶,人工核酸水解酶,dna切断剂;生物制备过程中转基因物质的去除。
细胞激活剂:由茉莉酮酸酯、苯并噻二唑等衍生的细胞激活剂;用于人参、紫杉烷、灵芝的次生代谢物的增产与调控。
生物转化:酵母、植物细胞催化或导致的刚性芳香与杂环化合物的硝基及酮立体选择性还原,化学选择性还原。
chem. commun. -2006-865: baker's yeast-mediated enantioselective reduction of substituted fluorenones
chem. commun. -2005-2338: a novel strategy for the preparation of arylhydroxylamines: chemoselective reduction of aromatic nitro compounds using bakers' yeast
chem. commun. -2004-2339: a novel strategy for the preparation of arylhydroxylamines: chemoselective reduction of aromatic nitro compounds using bakers’ yeast
tetrahedron-asymmetry-2010-825:
tetrahedron lett. -2004-1247: naphthalimide-thiazoles as novel photonucleases: molecular design, synthesis, and evaluation
appl. microbio. biotech. -2006-298: efficient induction of ginsenoside biosynthesis and alteration of ginsenoside heterogeneity in cell cultures of panax notoginseng by using chemically synthesized 2-hydroxyethyl jasmonate
appl. microbio. biotech. -2006-164: novel synthetic 2,6-dichloroisonicotinate derivatives as effective elicitors for inducing the biosynthesis of plant secondary metabolites
appl. microbio. biotech. -2005-98: a novel synthetic fluoro-containing jasmonate derivative acts as a chemical inducing signal for plant secondary metabolism
bioorg. med. chem. lett. -2005-1769: novel 2-aminothiazonaphthalimides as visible light activatable photonucleases: effects of intercalation, heterocyclic-fused area and side chains
bioorg. med. chem. lett. -2003-3513: thiadiazole: a new family of intercalative photonuclease with electron transfer and radical mechanisms
bioorg. med. chem. lett. -2004-2665: highly-efficient dna photocleavers with long wavelength absorptions: thio-heterocyclic fused naphthalimides containing aminoalkyl side chains
bioorg. med. chem. lett. -2004-2335: n-aroyloxylthioxo-naphthalimides as dna photocleavers of aroyloxyl oxygen radicals: synthesis, evaluation, and substituents' effect
bioorg. med. chem. lett. -2004-4755: novel fluoro- and hydroxyl-containing jasmonate derivatives as highly efficient elicitors in suspension cultures of taxus chinensis
bioorg. med. chem. lett. -2006-803: hydrolysis of plasmid dna and rna by amino alkyl naphthalimide as metal-free artificial nuclease
bioorg. med. chem. -2006-2935: novel fluorescent markers for hypoxic cells of naphthalimides with two heterocyclic side chains for bioreductive binding
bioorg. med. chem. -2006-23: eliminating nucleic acids contaminants by hydrogen peroxide-induced free radicals during the preparation of proteins
biotech. bioeng. -2005-516: highly efficient strategy for enhancing taxoid production by repeated elicitation with a newly synthesized jasmonate in fed- batch cultivation of taxus chinensis cells
biotech. bioeng. -2004-809: novel chemically synthesized hydroxyl-containing jasmonates as powerful inducing signals for plant secondary metabolism
biotech. bioeng. -2004-595: novel synthetic jasmonates as highly efficient elicitors for taxoid production by suspension cultures of taxus chinensis
biotech. progr. -2006-331: novel chemically synthesized salicylate derivative as an effective elicitor for inducing the biosynthesis of plant secondary metabolites
biotech. and bioprocess engi. -2005-162: efficient elicitation of ginsenoside biosynthesis in cell cultures of panax notoginseng by using self-chemically-synthesized jasmonates
chem. res. chinese univ.-2010-981:
biochem. engineering. j. -2006-23: eliminating nucleic acids contaminants by hydrogen peroxide-induced free radicals during the preparation of proteins